By: Lonna Gordon
Process Engineering
IPS- Integrated Project Services, LLC
Solids transfer is typically considered a problem reserved for the oral-solid dosage (OSD) community, where the powder is the main product bulk; however, it comes up with reasonable frequency in the biotechnology world as well. Applications range from adding salts to a sterile media tank, dry media preparation, pH adjustment after filtration processes, as well as addition of precipitation salts to a tank. This article will survey the basics of closed powder transfer for the biotech professional.
The primary assumption we will make is that the powder or salt is pre-weighed and measured into a powder transfer container. If the solids must be metered into the process, this level of complexity is beyond the scope of this piece. The second assumption is that closed-transfer is desired for protection of the product. Our third assumption is that powder quantities are too large to use a single-use bag and manual lifting as the transfer method. Larger quantities will be moved through the facility in drums or Intermediate Bulk Carrier, and demand more specialized equipment for successful transfer into the processing vessel.
In order to ensure that the transfer is fully enclosed, the primary connection between the powder-supply container and the receiving process vessel will be through an SIP capable split-butterfly valve (SBV). Most commonly used for powder transfer applications in OSD facilities, this specialized valve allows two separate vessels to create a clean, closed transfer connection. However, there are only a few SBVs that can be equipped with a sprayball. This a detail to consider when selecting a valve.
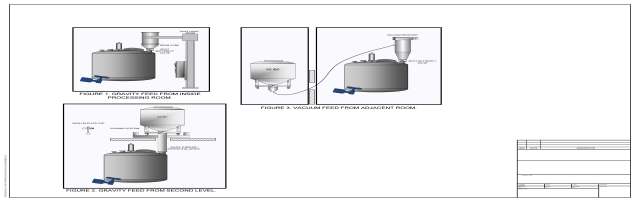
There are two ways to transfer the powder from the container to the vessel: gravity feed and vacuum transfer. Gravity feed involves positioning the entire container above the vessel, opening the connection between them, and allowing the solids to pour down into the vessel. With vacuum transfer, the drums or IBC can be located at any level relative to the tank. The powder is moved via negative pressure to a receiver mounted on the vessel, where it is then dropped into the vessel through the connecting valve.
Both options allow for the powder container to be located either in the processing suite with the vessel or in a separate room.
Gravity Feed – In-Room Transfer
A hoist is used to lift the drum or IBC above the tank and slew or invert it into position. It mates with the SBV. The hoist can be permanently fixed in the room, or it can be a mobile unit that carries the drum into the room and is stored elsewhere at all other times. The elevation available in the room is the limiting factor when using this method.
Gravity Feed – Second-Floor or Mezzanine Transfer:
The drum or IBC can also be lifted to a second floor or mezzanine using an elevator and docked to a transfer chute that connects to the vessel below. If a completely separate second-story space is used for docking, it can be a lower classification than the vessel’s processing suite. This is due to the connection to the chute will be totally enclosed, and there will be no contact between the contents of the IBC and the surrounding environment. There are some cleaning considerations for the chute; it will require a sprayball cap to wash it in place before removal for cleaning. Additionally, there must be space available for utilization on multiple floors.
Vacuum Transfer:
Vacuum transfer can occur from almost any space adjacent to the receiving vessel – the same room, the next room, the floor above, or the floor below. For IBCs, an adapter on the outlet of the IBC allows the powder to be pulled through a transfer hose to a receiver at the top of the tank. The transfer is completely enclosed and therefore, the IBC can be stationed in a room of any classification.
Drums, on the other hand, typically require an operator to manipulate a wand in the drum to ensure full powder transfer. This process is not completely enclosed and should take place in a space with a classification that matches that of the receiving vessel. There may be some fully closed, more customized options available for drums as well.